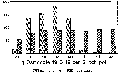 Click image for larger view.
Click image for larger view. Figure 2. The effect of Cycocel on severity of three bacterial diseases of Hibiscus rosa sinensis.
Return to: CFREC Home Page
Return to: CFREC Research Index
University of Florida, IFAS
Central Florida Research and Education Center - Apopka
CFREC-Apopka Research Report, RH-91-4
A. R. Chase *
Pest management strategies for hibiscus diseases have been researched during the past ten years at the CFREC-Apopka. Producers of hibiscus and other ornamentals face the challenge of producing a variety of blemish-free crops. Due to the special conditions of greenhouse production, however, they have a wide range of management techniques available for both fungal and bacterial disease control (Table 1).
GENERAL METHODS
Exclusion
The most effective strategy of preventing a pathogen from infecting plants is exclusion by quarantine. This is usually done in situations where the pathogen is very destructive and is not present in a particular geographic area. Growers should implement their own quarantine measures to safeguard against introduction of a pathogen. When new plants are purchased, they can be maintained in separate structures until their health is confirmed. It is especially important to maintain stock plants under quarantine to protect them from infections, since contaminated stock plants produce only contaminated cuttings.
Sanitation
Pathogens may be introduced in plant propagules, potting media, pots, equipment, insects and weeds, soil under benches and in walkways, and sometimes irrigation water. The specific control method which will be most effective depends upon the source of the pathogen. Eliminating greenhouse weeds and crop debris, removing diseased plants, and occasional removal of diseased leaves, collectively create an environment unfavorable to pathogen spread and favorable to early disease detection. Benches should be cleaned and sanitized between crops to eliminate contamination of new crops. Use of clean pots, flats and tools is important. If benches are cleaned between crops, placing new plants in contaminated pots or flats will negate the benefit of all other control strategies. Recognition and removal of diseased plants can aid in decreasing spread of disease between plants. Removal of infected leaves or entire plants is also a common practice for control of some diseases which greatly reduces inoculum available for future disease outbreaks.
Growing media
Growing media are an integral part of all plant production systems for greenhouse ornamentals. Media must be mixed or held in areas which are not contaminated with native soil. The media should be covered with a water-proof tarp to reduce chances of contamination with pests such as insects, weeds and plant pathogens and exposure to rainfall. Growing plants on raised benches removes them from a very common and extremely important source of infection, the ground. Many root and stem rot pathogens are present in native soil and move into ground beds and pots placed on the ground.
Irrigation
One of the most important considerations in irrigation management is elimination of standing water on plant foliage since this is necessary for infection to occur in most bacterial and fungal leaf diseases. Water management encompasses the water needs of the crop plant as well as the delivery method and humidity. Water stress through either too much or too little water can result in more severe disease development. This is often seen in root rot diseases caused by pythiaceous fungi which are more serious when plants are over-watered. Roots die when kept too wet due to oxygen starvation. Once the pathogen gains entrance through dead roots it can spread throughout the root system. The potting medium should not retain excessive water for long periods of time since even a small amount of water can be too much. Irrigation management of plants in a heavy potting medium is even more critical because it holds more water and has fewer large pores for air, an ideal condition for root disease.
The method of delivering water to the plants is also an important consideration for reducing both disease development and spread. Water systems which cause leaves to stay wet for long periods of time or cause splashing are ideal ways to spread pathogens and increase disease severity. Rapid leaf drying greatly reduces disease severity since many fungal spores and bacteria cannot infect leaves without a minimum period of free water on leaves. It is even better to use a method of irrigating which does not deliver water to the leaf surface at all. Systems such as drip irrigation, ebb and flow systems, and capillary mats are utilized for a variety of plants to minimize pathogen spread and disease development.
Biological control
The potential for use of biological control agents in greenhouse ornamentals is relatively high for insects and mites but not for many pathogens. Unfortunately, the zero tolerance level for damage on the ornamental product indicates that use of biological control may be confined to stock areas or to early stages of crop production where minimal damage may be acceptable. Another problem with implementing biological control in the greenhouse is the common use of a multitude of pesticides which can eliminate non-target biological control agents as effectively as the target pest. Biological control is not currently a method which can be used to control most diseases of greenhouse ornamentals. Although some examples of successful biological control of a plant disease are available they have not been readily employed commercially since they are specific to a particular disease on a relatively small group of crops.
Pathogen-free plants
Most potted flowering plants are produced from cuttings of tips or stems which can be contaminated with a wide variety of fungi, bacteria and viruses. Utilization of pre-plant dips to control diseases is usually ineffective for a variety of reasons. First, the chemicals which are available are not eradicants and the degree of control is less than 100% even under ideal conditions. Second, the act of immersing cuttings in a water solution creates ideal conditions for pathogen spread. Even a very low incidence of contamination or infection in dipped cuttings can result in contamination of all cuttings. Finally, immersing cuttings with latent infections into water can trigger the development of many diseases.
Establishment of pathogen-free blocks of mother or stock plants is an important step toward eliminating introduction of contaminated cuttings. Pathogen-free plant propagules are available for some plants from tissue-culture. While some growers utilize pathogen-free tissue-cultured plantlets as a propagative material others use these plants to establish blocks of stock plants to produce cuttings. These blocks must be maintained under quarantine type conditions to keep them as free of plant pathogens as possible. Stock plantings should have a limited life to maintain both high productivity and pathogen-free status.
SPECIfIC METHODS TESTED FOR HIBISCUS DISEASES
Temperature
Knowing the optimal temperatures for development of a given disease is better used for timing control measures rather than altering the growing environment. Sometimes this information is helpful to disease diagnosticians since they can make special effort to look for target pathogens at certain times of the year. Hibiscus produced in Florida are subject to three bacterial leaf spot diseases which occur during different times of the year (Table 2). During the winter months, Pseudomonas syringae pv. hibisci occurs with Pseudomonas cichorii common during the fall and spring. In general, only Xanthomonas campestris pv. malvacearum is active during the summer months. Scouting for diseases only at times when temperatures are favorable allows for better management of personnel resources. In addition, preventive pesticide applications should be recommended only when disease development is possible and not on a year-round basis which is costly and potentially hazardous for the plants, the workers, and development of resistant populations.
Host nutrition
Host nutrition has been shown to affect severity of many diseases of ornamental plants. Both the rate of fertilizer and its source can affect disease development. Trials were conducted with hibiscus grown with different levels of slow-release fertilizer (Osmocote 19-6-12). Both plant quality and severity of Xanthomonas leaf spot were affected by fertilizer level (Fig. 1). Plant quality was very high when they received between 5 and 20 g of fertilizer per 5-inch pot.
In contrast, disease severity increased as fertilizer rate increased to the rate of 10 g per pot, then showed a rapid decline in severity above the 12.5 g rate (Fig. 1). Use of 15 to 20 g of fertilizer per pot resulted in excellent quality plants with very low levels of Xanthomonas leaf spot.
Host resistance
Resistant varieties should be used since they can be produced in the presence of the pathogen with a minimum loss to disease. The available information falls short of answering most questions concerning disease resistance of hibiscus but some information on four diseases common to Florida hibiscus is available (Table 3). Although American Beauty is relatively resistant to Phytophthora and both Pseudomonas leaf spots, it is highly susceptible to Xanthomonas leaf spot (Table 3). Most other cultivars tested were also resistant to some pathogens while susceptible to others. Choice of cultivars could be made on the basis of disease resistance as long as all diseases were not common in the nursery.
Pesticides and growth regulators
Pesticide usage remains the backbone of control for many severe diseases, due to the need for very high quality ornamental products. Some excellent pesticides are labeled for control of many diseases common to hibiscus with the exception of those caused by bacterial pathogens. Research has centered on controlling these diseases with fosetyl aluminum (Aliette 80WP), bromine treated mist (Agribrom) and the growth regulator chlormequat chloride (Cycocel) (Fig. 2).
Aliette 80WP has been shown to greatly reduce a variety of Xanthomonas diseases of ornamentals. However, when a test was completed on hibiscus inoculated with Xanthomonas the use of Aliette appeared to increase disease severity. In the same trial, cupric hydroxide was ineffective against Xanthomonas leaf spot but did not increase disease severity.
Another serious disease of hibiscus cuttings is caused by a variety of bacteria. Since this disease occurs on cuttings rooted under mist the use of water treatment compound such as Agribrom seemed appropriate to evaluate. Xanthomonas leaf spot was controlled 100% when 55 ppm of bromine was delivered through the mist system once/30 min 12 hours/day. Unfortunately, bromine toxicity was noted as necrotic spotting and shot hole when this rate was applied. A rate of 25 ppm bromine gave 76% control of Xanthomonas leaf spot and was apparently safe on well established cuttings. Further testing on unrooted hibiscus cuttings revealed that even 25 ppm caused delayed rooting and shoot development.
Grower observations regarding use of Cycocel on hibiscus led to the following research on bacterial disease control. Plants were treated with Cycocel at the recommended rate three times on weekly intervals prior to inoculation with a bacterial pathogen. Regardless of cultivar (10 were employed) or pathogen (Ps. cichorii, Ps. syringae, or X c. pv. malvacearum) the Cycocel treated plants were resistant to the bacteria (Fig. 2).
*Professor, Plant Pathology, Central Florida Research and Education Center-Apopka, 2807 Binion Road, Apopka, FL 32703-8504.
1. Chase, A. R. 1986. Comparisons of three bacterial leaf spots of Hibiscus rosa-sinensis. Plant Disease 70:334-336.
2. Chase, A. R., L. S. Osborne, J. M. F. Yuen and B. C. Raju. 1987. Effects of growth regulator chlormequat chloride on severity of three bacterial diseases of 10 cultivars of Hibiscus rosa-sinensis. Plant Disease 71:186-187.
3. Chase, A. R. 1989. Effect of fertilizer rate on susceptibility of Hibiscus rosa-sinensis to Xanthomonas campestris pv. malvacearum. Biological & Cultural Tests for Control of Plant Diseases 4:83.
4. Chase, A. R. 1989. Aliette 80WP and bacterial disease control - I. Xanthomonas. Foliage Digest 12(11): 1-3.
5. Chase, A. R. 1990. Control of some bacterial diseases of ornamentals with Agribrom. Proc. Fla. State Hort. Soc. 103: (In Press).
6. Jones, J. B., A. R. Chase, B. C. Raju and J. W. Miller. 1986. Bacterial leaf spot of Hibiscus rosa-sinensis incited by Pseudomonas syringae pv. hibisci. Plant Disease 70:441-443.
7. Lockhart, B. E. L. 1987. Evidence for identity of plant rhabdoviruses causing vein yellowing diseases of tomato and Hibiscus rosa-sinensis. Plant Disease 71:731-733.
8. Semer, C. R. IV. and B. C. Raju. 1985. Phytophthora leaf spot of Hibiscus, a new disease caused by Phytophthora parasitica. Plant Disease 69: 1005-1006
9. Uchida, J. Y. and P. S. Yahata. 1989. Hibiscus blight caused by Athelia. Hawaii Coop. Ext. Serv. Commodity Fact Sheet HIB-4(A). 2pp.
| Strategy | Fungi | Bacteria |
| Exclusion | moderate | high |
| Sanitation | high | high |
| Growing media | high | moderate |
| Irrigation | high | high |
| Biological | none at present | none at present |
| Pathogen-free plants | high | high |
| Temperature | not known | high |
| Host nutrition | not known | moderate to high |
| Host resistance | moderate | moderate |
| Pesticides | high | moderate |
Table 2. Optimal temperatures for development of some diseases of hibiscus.
| pathogen | °C | °F |
| Erwinia spp. | 28 - 34 | 77 - 94 |
| Phytophthora parasitica | 20 - 22 | 68 - 72 |
| Pseudomonas cichorii | 21 - 27 | 70 - 81 |
| Pseudomonas syringae pv. hibisci | 15 - 18 | 60 - 65 |
| Rhizoctonia solani | 20 - 35 | 68 - 95 |
| Xanthomonas campestris pv. malvacearum | 24 - 33 | 75 - 92 |
| Cultivar | Phytophthora | Ps. cichorii | Ps. syringae | Xanthomonas |
| American Beauty | low | low | low | high |
| Brilliant Red | moderate | low | high | low |
| Butterfly | not tested | low | low | moderate |
| Euterpe | not tested | low | moderate | low |
| Holiday | not tested | low | high | not tested |
| Painted Lady | high | low | high | moderate |
| Pink Versicolor | high | low | low | high |
| President | not tested | low | low | moderate |
| Senorita | moderate | low | moderate | high |
| White Red Eye | not tested | low | low | moderate |
Figure 1. The effect of fertilizer rate on top quality of Hibiscus rosa-sinensis and severity of Xanthomonas leaf spot caused by X campestris pv. malvacearum.
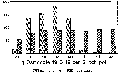 Click image for larger view.
Click image for larger view.
Figure 2. The effect of Cycocel on severity of three
bacterial diseases of Hibiscus rosa sinensis.