

This could be either one. They look identical!
CURRENT Updates available by writting to:
Biotype-Q update PDF FILE
Unfortunately, we have a developing whitefly issue in Florida. We are having major issues managing 2 biotypes in a number of areas in South Florida. Both biotypes are referred to as Bemisia tabaci. The Q biotype has been detected in a number of landscapes in Palm Beach County. This is the VERY FIRST TIME it has been found in a landscape or outside a greenhouse or nursery since it was found on an ornamental plant in a greenhouse many years ago (2004-2005). This is extremely troubling considering the issues we have with many of the tools we use to manage whiteflies.
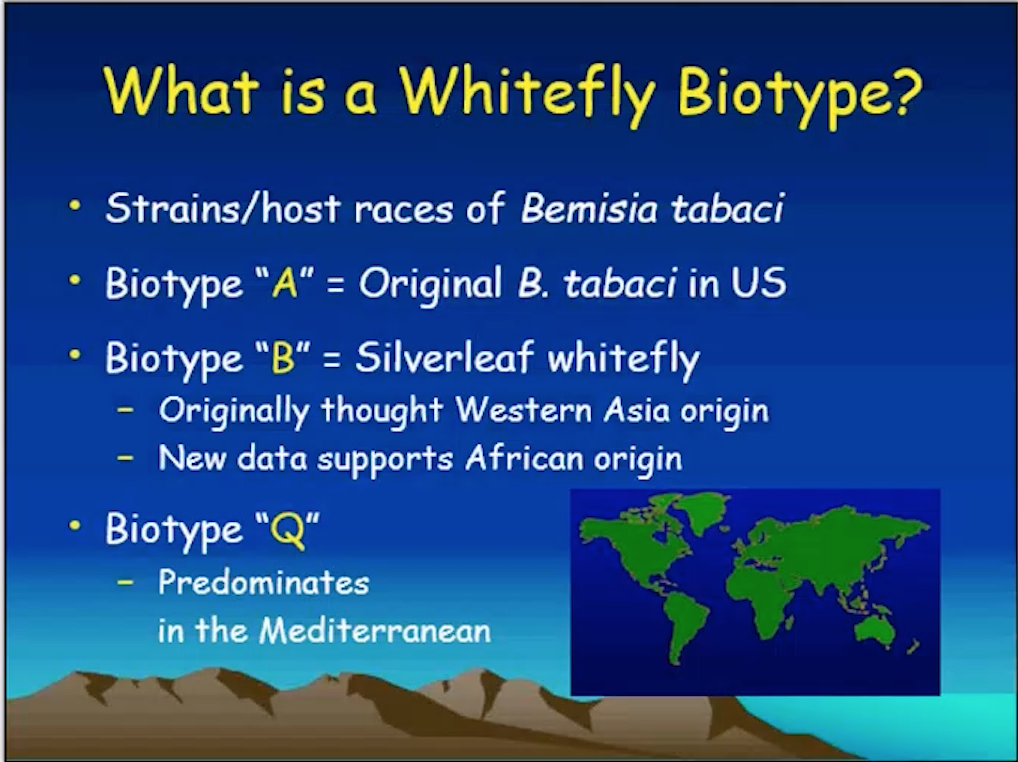
Bemisia tabaci (Gennadius) feeds on more than 600 host plants and vectors over 111 plant virus species and is considered to be a major invasive species worldwide. The taxonomic status of B. tabaci remains debated between 36 previously identified biotypes and the newly proposed 26 discrete species and they can only be identified by performing genetic analysis. Losses in agricultural production have increased owing to B. tabaci as new, more virulent and less pesticide-sensitive cryptic species have spread to all continents except Antarctica. Very few countries have escaped its cosmopolitan distribution and subsequent establishment of at least one of the B. tabaci cryptic species. The two most invasive members of the cryptic species complex posing the greatest threat to growers are Middle East –Asia Minor 1 (MEAM1) and Mediterranean (MED) (commonly known as biotypes B and Q respectively).
After the introduction of MEAM1 into the United States around 1985, unprecedented losses began occurring on poinsettia in the late 1980s in Florida, followed by high infestations in field-grown tomato crops. MEAM1 rapidly spread across the southern United States to Texas, Arizona and California, where extreme field outbreaks occurred during the early 1990s on melons, cotton and vegetable crops. Losses exceeded more than 500 million dollars in one year.
Indistinguishable morphologically from MEAM1, MED is extremely problematic to agricultural production because populations are highly prone to develop resistance to insect growth regulators (IGRs) and neonicotinoid insecticides. Both classes of insecticides are widely used for controlling whiteflies in many cropping systems, including cotton, and ornamentals. Based on recent reports, we may be in for a challenging year for whitefly management. We are receiving reports from the keys to Palm Beach County that whitefly populations in landscapes are reaching unprecedented levels and they don’t seem to be responding to pesticide applications. At this point in time, the Q-biotype has been found in 16 retail outlets, 7 wholesale nurseries and 10 residental landscapes. These detections have been in Broward, Duval, Highland, Hillsborough, Martin, Miami-Dade, Palm Beach, Pinellas and Seminole counties. Samples from all the other difficult to manage populations are the B-biotype.
Bemisia feeds on a large number of hosts. Click Here to download a PDF file that contains a list of Florida plants on which this whitefly has been found.
As part of National Invasive Species Awareness Week, the University of Florida, Auburn University, University of Georgia Center for Invasive Species and Ecosystem Health, and Southern IPM Center presented a number of webinars that on important invasive speices (Click Here). Two are relevant to the invasion of the United States by Biotype-Q of Bemisia tabaci.

NORTH AMERICAN Q-BIOTYPE INVASION PROJECT (41:32) (Click Here).

MANAGING THE US INVASION OF CRYPTIC WHITEFLY SPECIES: Q VS. B WHITEFLIES (39:14) (Click Here).
Agent Webinar (Click Here).
<p class="pSampling Whiteflies for Biotyping Click Here
Task Force Letter to Growers Click Here
An Important Message About Insects in the Landscape Click Here
A Letter to the Landscape Management Industry Click Here
Whitefly Management Program Click Here
Products Table Ent Whiteflies edited by LSOsborne Click Here
Landscape Pesticides with Activity on the Q Click Here
Invasion and Response: Impacts of Bemisia on Worldwide Agriculture. Click Here
RETURN TO THE WHITEFLY PHOTO PAGE Click Here
THE Q-BIOTYPE whitefly, a New Whitefly in Florida: A guide for homeowners Click Here
Palm Beach Whitefly Task Force Click Here This website is particularly helpful. A task force was organized a number of years ago that helped with the management of invasive whiteflies that plagued many communities in South Florida. It is a great example of how the community (garden club, city officials, pest management companies, consultants, County Extension, USDA-ARS and University of Florida scientists) work together to solve a problem. This effort is totally responsible for the detection of the Q-biotype in the landscape. We have a very well trained and cooperative group of consultants and private pest management personnel working with us in Palm Beach County. The county extension agent for Palm Beach has also been instrumental in the detection and management of this new pest. As our cooperators find difficult to manage populations of Bemisia they have sent them in for biotyping. Once identified they have worked with us and even their competition to manage the situation!
General Information:
Whiteflies have long been considered a major pest of ornamental crops. Until 1986, the primary pest species was the greenhouse whitefly (GHWF), Trialeurodes vaporariorum (Westwood). In 1986, Bemisia tabaci (Gennadius) was found attacking an array or ornamental plants in Florida greenhouses. Scientists in Florida soon realized that this species was causing damage different than any ever attributed to whiteflies. This damage caused many plants to show signs of being infested because the plants turned yellow, white or silver depending on the specific host plant. Because various squash species tuned silver when infested with the B-biotype (also known as Bemisia argentifolii Bellows & Perring) this biotype was given the common name, silverleaf whitefly (SLWF). Throughout the following discussion we will refer to this whitefly as either the B-biotype of Bemisia tabaci or SLWF.
The Q biotype--In March 2005, Dr. Tim Dennehy (University of Arizona) reported the detection of the Q-biotype of Bemisia tabaci. This detection came after testing whiteflies collected from poinsettia in a retail outlet. These whiteflies were collected in December of 2004 as part of a pesticide resistance monitoring program in Arizona. Most of the samples had been from cotton and other crops, poinsettia and ornamental crops were not the primary focus of this monitoring program. Drs. Judy Brown, Tim Dennehy (University of Arizona) and Frank Byrne (University of California) independently verified the whitefly as being the 'Q-biotype'. This was the first time this particular strain had been found in the United States. The Q-biotype is thought to have originated from the Mediterranean region and has been associated with whitefly control problems. Dr. Dennehy determined that the strain of whiteflies collected from the poinsettia (Poinsettia-04) could be characterized as being virtually immune to the IGR pyriproxyfen (Distance), having strikingly reduced susceptibility to the IGR buprofezin (Talus) and a reduced susceptibility to the neonicotinoids insecticides imidacloprid (Marathon or Merit), acetamiprid (TriStar) and thimethoxam (Flagship).
It must be noted, that this information is generated using laboratory bioassays and that no field efficacy work has been conducted to determine how these data relate to controlling the Q-biotype in the field, at least in the United States. . However, significant research has been conducted to determine which materials can be used to manage this biotype on ornamentals in greenhouses (reflected in the management plan (PDF). This biotype is known to have resistance to pyriproxyfen (Horowitz et al. 2003), buprofezin and reduced susceptibility to the neonicotinoid insecticides imidacloprid and acetamiprid in other regions of the world.
Description and Biology
The greenhouse and silverleaf whiteflies are the primary whitefly pests of greenhouse crops. Banded-winged and citrus whiteflies are also found in greenhouses but usually do not reproduce and develop damaging populations. Whiteflies, when compared to other pests of ornamentals, have a long life cycle, ranging from 2.5 to 3 weeks up to 2 months under cooler conditions. Adults are moth-like and covered with white, waxy powder. Adult female whiteflies are about 1/16 of an inch in length and deposit about 50 eggs in cool environments and up to 400 eggs at higher temperatures. Consequently a whitefly population can reach very high levels in a few generations at higher temperatures. Eggs are inserted on end upon a short stalk on the underside of leaves. The eggs are whitish to light beige but darken to a dark blue or purple before hatching. The immature stages resemble miniature scale insects. They are flat and oval, glassy to opaque, light yellowish or greenish, and often provided with a fringe of wax filaments. Older immatures tend to be darker and either cream or yellow. Newly hatched immature crawlers move around on the leaf for only a few hours and then insert their mouthparts and begin to feed. The remainder of the immature development is sessile. Whiteflies feed exclusively on leaves, nearly always occurring on the undersurface. They suck juices from the plants and also excrete large quantities of honeydew in which sooty mold grows.
Feeding Damage and Symptoms
Whiteflies feed on more than 500 species of host plants. Greenhouse-grown ornamentals such as poinsettia, hibiscus, ivy, gerbera daisy, lantana, verbena, garden chrysanthemum, salvia and mandevilla are especially susceptible to whitefly damage. Whiteflies feed on plant phloem by injecting enzymes and removing the sap, reducing the vigor of the plant. Honeydew secretions from the whitefly promote the growth of sooty mold which also significantly reduces plant quality. The most obvious whitefly feeding damage symptoms are stem blanching, chlorotic spots, leaf yellowing and shedding and, at high population levels, plant death. In many crops the damage caused by Bemisia tabaci is indirect. This species of whitefly is responsible for transmitting many devastating viruses.
Detection and Sampling
Monitor whitefly population levels by trapping winged adults on sticky cards and inspecting leaves for the presence of feeding immatures. Strategically place yellow sticky cards throughout the greenhouse, especially near doors and among new plants to provide information about the presence and movement of whiteflies. Detect whiteflies on plants by randomly selecting 10 plants per 1,000 square feet of greenhouse space and thoroughly examining these plants on the underside of leaves, using a 10X hand lens, for the presence of whitefly adults, nymphs and eggs. Determination of biotypes is accomplished in the laboratory by using sophisticated biochemical techniques. Therefore, growers will have to send preserved whiteflies to one of the participating labs. Currently, this pest is not rated as a pest with quarantine status which means there are no legal requirements for these labs to report their findings. Irrespective of which whitefly biotype you have it's prudent to monitor for whiteflies and develop a Resistance Management Program (RMP).
Management
Chemical Control. Insecticides are the primary method used to control whiteflies. Many compounds have been used, but the systemic Marathon (imadacloprid) has been used most frequently over the past few years. The newer chemically related compounds Celero (clothianidin), Flagship (thiamethoxam), Safari (dinotefuran), and TriStar (acetamiprid) are also effective. Endeavor (pymetrozine), Aria (flonicamid), and the insect growth regulator (IGR) Distance (pyriproxyfen) are new chemical classes that have activity as well. There are several other effective IGRs: Azatin/Ornazin/AzaDirect (azadirachtin), Enstar II (kinoprene), Pedestal (novaluron), and Talus (buprofezin). The newest material to be registered for whitefly control is Judo (spiromesifen). Tank mixes of orthene (acephate) with a pyrethroid are synergistic, providing better control than either alone. A few other general insecticides, aerosols and soaps or oils can also be used. The newly hatched crawlers and the adults are most susceptible to chemicals, but the waxy covering on the larger immatures makes them more difficult to cover thoroughly with spray material. Resistance is a PROBLEM and every effort should be made to rotate chemicals each time an application is made don’t rely on any one product or chemical class for whitefly control.
Exclude whiteflies from the greenhouse. Whiteflies are very small. Screens with a hole size of <0.19mm are required to exclude adult whiteflies.
Be proactive, find them first. Scout greenhouses by using sticky cards, leaf inspections or random sampling techniques. Use this information as a basis for decisions for chemical applications. Start any control practice early, when the first whiteflies are detected. Control is difficult once populations have increased to high levels.
Biological Control. Several biological agents are available including predators (i.e. Orius, Delphastus, lacewing larvae, etc.), parasitoids (i.e. Eretmocerus, Encarsia, etc.) or pathogens (i.e. Beauveria bassiana, etc.). Check with suppliers on compatibility with chemicals and environmental requirements such as temperature, humidity, and daylength. In Florida, Bemisia tabaci is effectively managed on ornamentals and vegetables grown in greenhouses with Encaria transvena. Other potential management tools that you probably are not currently using. One area where nothing is currently being done to manage pests is during transit or shipping of plants. Drs. Lindquist, Oetting, and Osborne obtained significant whitefly reduction by treating whitefly infested cuttings and plants with insect pathogens just prior to boxing and shipping. A large grower could easily produce their own insect pathogens that would be used throughout the growing season in conjunction with all of the other pesticides being used. A couple of the largest growers in Florida are currently doing this. With fungi, it is not a typical case of using either chemical or biological control. YOU CAN USE BOTH ECONOMICALLY.
WHAT CAN YOU DO?
First of all, don't panic! Growers that have the Q-biotype in other parts of the world are still producing crops.
Go Back To The Basics And Fine Tune Your Whitefly IPM Program!
Scout- Determine the extent of any whitefly problems. Make sure everyone’s training is up to date and that employees are sensitized to the fact that not all whiteflies are the same. If any populations exist and the numbers or even their presence seems the least bit unusual FIND OUT WHAT YOU HAVE. Studies have shown that in a population that has both Q and B biotypes, increasing pesticide applications will only increase the proportion of the population that are the Q biotype.
Prevention- Don’t let new whiteflies into your operation. You may not be able to prevent movement into your nursery from outside sources but reduce your risk by inspecting new plant material in a secure place so that whiteflies don’t escape. Quarantine all new plant material introduced into your nursery.
Sanitation- Remove sources of infestation that might carry over populations from one season to the next.
Cultural- Grow plants so as to facilitate good pesticide coverage. If possible, try to have a crop free period so as to break any cycling within your nursery.
Physical- Screening, weeding…
Resistance management- a numbers game?
The greater the number of whiteflies present when an application is made the greater the chance that at least one individual might posses the ability to survive the treatment.
The more frequent a given pesticide or mode of action is used the greater the potential for developing a problem. Along those same lines, the longer the residual activity the greater the “selection” pressure.
If no virus is involved in the system, then the ultimate goal is not zero whiteflies throughout the production cycle but zero whiteflies on the plant material leaving the facility. If this statement can be agreed to, then we have a much better chance managing resistance in whiteflies.
Older recommendations stated that “Insecticides should be applied a minimum of two times at a five to seven day interval to allow for egg hatch between applications so that both adults, nymphs and individuals that hatch from eggs are killed. This is not appropriate for many of the new pesticides that have residual activity of one week or greater. If the insecticide is properly applied and is not providing control, change to another material with a different mode of action because whitefly populations have the propensity to develop resistance. This is why scouting weekly and especially after a pesticide application is critical.
Growers must learn from experience which chemicals, when correctly applied, fail to give satisfactory control, and to then try other materials in a different classification.
Often, a severe pest problem occurs on plants in the retail shop because a few eggs, nymphs or adults survived even the best and most conscientiously followed control program during production. It is imperative, therefore, that your most effective pesticide be applied to major whitefly host plants as close to the date of shipment as possible.
There are a number of ways to deal with this issue but the bottom line is; the fewer applications one makes of materials from a given class, the smaller the potential for resistance developing. To that end, what can be done? First off, we recommend you develop a list of all the pesticides that are legal to use for whitefly control on the crop you are growing. Next, we suggest that each be evaluated under your particular situation for phytotoxicity. When you are finished you will have a list, hopefully not too short, from which you can develop a management program. The next problem is to review the labels to find restrictions/limitations on how often a material can be applied to a given crop. The plan you put together should be based on all of these points and the fact that growers will have to apply materials to manage other pests. Scouting is essential to the success of any pest management program.
From a resistance management perspective it would seem prudent to treat with those materials highlighted with yellow when the whitefly pressure and numbers are the greatest. Once the population have been reduced then treatment with those materials highlighted with green could be used. The theory behind this is simple: DON'T USE YOUR BEST MATERIALS AGAINST LARGE POPULATIONS BECAUSE THE LARGER THE POPULATION THE GREATER THE CHANCE OF TREATING A RESISTANT WHITEFLY.
The Mode of Action information was obtained from:
Insecticide Resistance Action Committee Mode of Action Classification (LINK)
Not all Q biotypes are going to respond the same to insecticides. The strain detected by Dennehy was shown to be highly resistant to a number of newer insecticides but this does not mean that all future Q biotypes detected in the US will be comparably resistant. The Q biotype detected by Dennehy has reduced susceptibility to neonicotinoid insecticides, including imidacloprid, thiamethoxam, and acetamiprid. However, this does not necessarily mean that these chemicals will fail to control the Q biotype, especially under the conditions of treating potted plants. Based on tests of Q biotypes conducted prior to the detection of the Q biotype in the US, it has been concluded the neonicotinoid insecticide dinotefuran is unaffected by the resistance of the Q biotype to imidacloprid and other neonicotinoids. However, this result has not yet been confirmed with the Q strain found in AZ. When Q biotypes are found in the US and “eliminated” with dinotefuran, or any other effective insecticide, this in no way insures that the Q has been eradicated at that location. There are few examples of eradication of polyphagous, parthenogenetic homopteran pests.
We will post updates on this website as well as send out news to the Bemisia-L Listserver.
BEMISIA-L Listserver
What is a list server? A list server is like on open conversation that travels through e-mail. One person e-mails a question to a specific email address and it is sent to all on the list. As people respond to the questions and statements they are e-mailed back to all on the list. This is a good way to get questions answered and to learn about the many new species of whiteflies attacking plants in Florida. This list is not limited to Bemisia. In fact, we will probably concentrate on a number of newly introduced invasive species.
To Subscribe
Send an mail to listserv@lists.ufl.edu
Leave the subject line blank and in the text of the message type the following:
Subscribe Bemisia-L "YOUR NAME GOES HERE"
DO NOT PUT "" AROUND YOUR NAME
To UNsubscribe
1. Send an mail to listserv@lists.ufl.edu
2. Leave the subject line blank and in the text of the message type the following:
Unsubscribe Bemisia-L or signoff Bemisia-L
Lance S. Osborne
407-461-8329
lsosborn@ufl.edu